

Articles | January 23, 2025
Our latest short quarterly insight for sponsors of group health plans focuses on biosimilar drug therapies.
It covers:
Biosimilar adoption has been slow. For medications with available biosimilars, generally less than 10 percent of prescriptions have been for a biosimilar, but the percentage increased significantly in Q2 2024 due to changes in pharmacy benefit manager (PBM) practices.
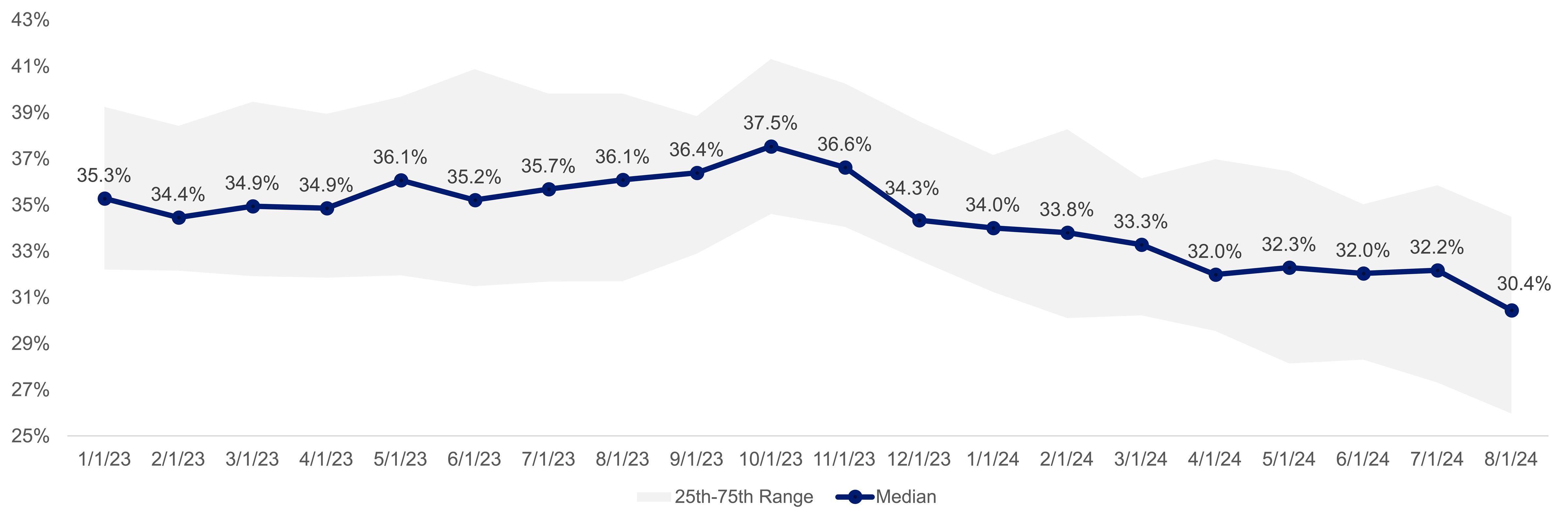
Source: Segal’s SHAPE data warehouse, 2024
The promise of Rx plan cost savings from competition of biosimilar drug therapies is still in its early stages. A biosimilar is like a generic version of a biologic drug. However, a generic drug is an exact copy of a brand drug. A biosimilar, on the other hand, is a biologic (medication derived from living organisms) that is similar to another biologic medication that is already licensed by the Food and Drug Administration (FDA), known as the originator or reference product, and is close enough that it leads to similar clinical outcomes.
Interchangeable biosimilars may be automatically substituted by a pharmacist for the original biologic or reference product, like generic substitution for brand-name medications, while non-interchangeable biosimilars require prescriber approval. Note that applicable laws vary by state.
Due to complexities in developing, manufacturing and storing these biologics, they typically have higher overall costs than non-biologic medications, and biosimilars may still be costly when compared to costs of generic medications. However, biosimilars, which were first approved in 2015, have shown roughly 35 percent savings over originator products when covered under medical benefits. In recent years, more biosimilars have been approved as alternatives to originator products that are commonly provided under pharmacy benefits.
As of January 2025, for example, Humira®, typically the top biologic medication in terms of pharmacy expenditures, has 10 FDA-approved biosimilars (several are considered interchangeable). Humira biosimilars represent up to 85 percent savings off of Humira's list price and could result in significant savings for plan sponsors, even when accounting for potential rebates.
Since the first biosimilars were approved, the promise to lower costs for plan sponsors has not fully materialized. This is likely due to market dynamics and the dominance of originators such as Humira. According to Segal’s SHAPE data warehouse, originators made up 99.6 percent and 98.9 percent of all biologics spending in 2022 and 2023, respectively.
In April 2024, CVS began excluding Humira from most of its major commercial formularies. This change resulted in a decrease of 2.3 percentage points in the median allowed cost of all biologics drugs through August 2024 in Segal’s SHAPE database. Beginning in 2025, ESI and OptumRx have also taken steps to remove Humira from their formularies.
There is a growing pipeline of biosimilars that will reshape various therapeutic areas, including oncology, diabetes and cardiovascular treatments.
Some reasons for the modest adoption rates include:
Despite challenges, biosimilars are projected to generate substantial cost savings as adoption increases. Plan sponsors can use several strategies to encourage use of biosimilars:
Consider these questions when developing strategies to promote biosimilars:
Contact your Segal consultant or get in touch with us.

Health, Multiemployer Plans, Public Sector, Healthcare Industry, Higher Education, Architecture Engineering & Construction, Pharmaceutical, Corporate, Mental Health

Health, Public Sector, Multiemployer Plans, Healthcare Industry, Higher Education, Architecture Engineering & Construction, Pharmaceutical, Corporate, Healthcare Cost Management

Health, Healthcare Cost Management
This page is for informational purposes only and does not constitute legal, tax or investment advice. You are encouraged to discuss the issues raised here with your legal, tax and other advisors before determining how the issues apply to your specific situations.
Don't miss out. Join 16,000 others who already get the latest insights from Segal.
© 2026 by The Segal Group, Inc.Terms & Conditions Privacy Policy Style Guide California Residents Sitemap Disclosure of Compensation Required Notices