

Archived Insight | December 15, 2020
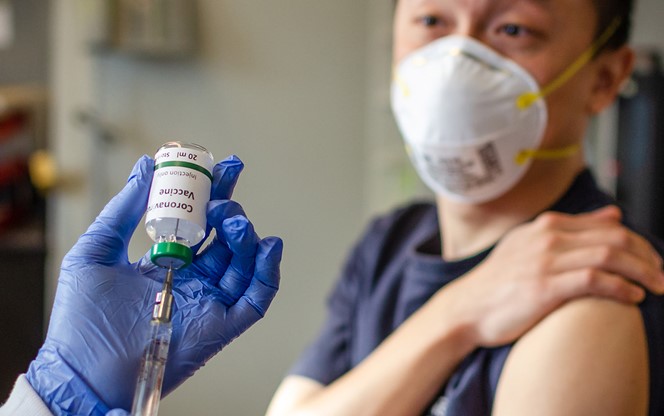
The U.S. Food and Drug Administration (FDA) has now authorized Pfizer’s COVID-19 vaccine for emergency use.
All group health plans that are non-grandfathered under the Affordable Care Act (ACA) must cover the vaccine without cost sharing.
The federal agencies have not yet stated when the “15 business day” time period for coverage must begin.
We're using January 1, 2021, as that is 15 business days after the vaccine was authorized, but the date could be changed in the future.
Share this page
Sponsors of non-grandfathered plans should prepare to cover Pfizer’s COVID-19 vaccine without cost sharing by January 1, 2021. To maximize access, Segal recommends that COVID-19 vaccines be covered under both the medical plan and the pharmacy benefit.


All plan sponsors should get ready to answer participants’ questions about the vaccine and where they can obtain it.
State and local public health departments will likely be the best source of information about where to obtain the vaccine in their community.
Although grandfathered plans are not required by federal law to cover COVID-19 vaccines (with or without cost sharing), plan sponsors of such plans should review how their plans currently cover vaccines and decide how to approach coverage for COVID-19 vaccines.


We cover background on the approvals and coverage. We also discuss approval of other COVID-19 vaccines.
On December 11, 2020, FDA issued the first emergency use authorization (EUA) for Pfizer’s COVID-19 vaccine, developed in conjunction with the German company BioNTech.
The Pfizer-BioNTech vaccine was approved for use for individuals 16 years of age and older. On December 12, 2020, the CDC’s Advisory Committee on Immunization Practices (ACIP) recommended use of that vaccine.
The director of the CDC adopted the ACIP’s recommendation on December 13, 2020.
The ACA requires non-grandfathered group health plans to provide certain preventive service without cost sharing. This includes services with an “A” or “B” recommendation from the U.S. Preventive Services Task Force (USPSTF) and immunizations recommended by the ACIP and adopted by the director of the CDC.
The CARES Act requires that COVID-19-related preventive services recommended by the USPSTF or the ACIP/CDC be covered by non-grandfathered group health plans (and any insurers of such plans) within 15 business days after any such recommendation is made. This is a much quicker deadline than applicable to other ACA preventive services. For COVID-19 vaccines, the 15-day clock began on December 13, 2020, when the director of the CDC adopted the ACIP’s recommendation.
The federal agencies have not yet stated when the “15 business day” time period begins. We are using January 1, as that is 15 business days after December 13, but the date could be changed in the future.
A recent interim final rule from the Departments of Treasury, Labor and Health and Human Services (the Departments) that took effect on November 2, 2020 implements these accelerated coverage requirements for COVID-19 preventive services, including vaccines, under the CARES Act.
Below we highlight significant parts of our summary of the interim final rule.
Plans and insurers must cover items or services that are “integral” to the furnishing of any required preventive service, including those related to COVID-19. This means that plans and insurers must cover recommended vaccines, as well as vaccine administration. At this time, the federal government is paying for the vaccine doses, and non-grandfathered group health plans and insurers must cover administration costs without cost sharing.
Non-grandfathered group health plans must cover COVID-19 preventive services without cost sharing whether provided by an in-network or out-of-network provider. This requirement applies to payment through both the medical and pharmacy benefits of the plan.
The requirement to cover out-of-network COVID-19 preventive services without cost sharing will sunset at the end of the current public health emergency (which will end January 21, 2021 unless extended again, as is likely to happen).
When provided by an out-of-network provider, the plan or insurer must reimburse the provider a “reasonable amount,” as determined in comparison to prevailing market rates for such services. The Departments note that they would consider the Medicare payment rates to be reasonable.
The Medicare payment rate for COVID-19 vaccine administration will be $28.39 to administer single-dose vaccines. For a COVID-19 vaccine requiring a series of two or more doses, the initial dose(s) administration payment rate will be $16.94, and $28.39 for the administration of the final dose in the series. These rates will be geographically adjusted.
Grandfathered group health plans are not subject to the ACA’s preventive benefit requirements and therefore not subject to these vaccine coverage rules. However, these group health plans may have coverage requirements in their plan documents, or may wish to adopt vaccine coverage benefits offered through their administrative services provider or pharmacy benefit manager. Plans would need to determine whether cost-sharing will be charged for the COVID-19 vaccine administration costs and if so how this will be administered.
Moderna’s COVID-19 vaccine will be the next candidate considered by the FDA (scheduled for December 17, 2020) and the ACIP/CDC. Next on the list will be the vaccines by AstraZeneca–Oxford and Johnson & Johnson. Each of their respective vaccines are in Phase 3 trials.
Many other vaccines are likely to follow in short order, and the coverage requirements explained above will apply to each one.
The FDA’s first analysis of the clinical data from the trials found that the coronavirus vaccines worked well regardless of a volunteer’s race, weight or age. According to the manufacturers, the protection provided by the neutralizing antibodies generated by these vaccines lasted for four months or beyond.
The ACIP also voted to include the vaccine in 2021 immunization schedules. Vaccine administration will be phased in accordance with ACIP and CDC recommendations, with health care workers and residents and workers in long-term care facilities being included in the first phase. The CDC will continue to issue technical guidance on vaccine administration.
We have answers.

Health, Compliance, Multiemployer Plans, Public Sector, Healthcare Industry, Higher Education, Architecture Engineering & Construction, COVID-19, Corporate

Health, Multiemployer Plans, Public Sector, Healthcare Industry, Higher Education, Architecture Engineering & Construction, COVID-19, Pharmaceutical, Corporate

Health, Multiemployer Plans, Public Sector, Healthcare Industry, Higher Education, Architecture Engineering & Construction, COVID-19, Pharmaceutical, Corporate, Mental Health
This page is for informational purposes only and does not constitute legal, tax or investment advice. You are encouraged to discuss the issues raised here with your legal, tax and other advisors before determining how the issues apply to your specific situations.
Don't miss out. Join 16,000 others who already get the latest insights from Segal.
© 2026 by The Segal Group, Inc.Terms & Conditions Privacy Policy Style Guide California Residents Sitemap Disclosure of Compensation Required Notices